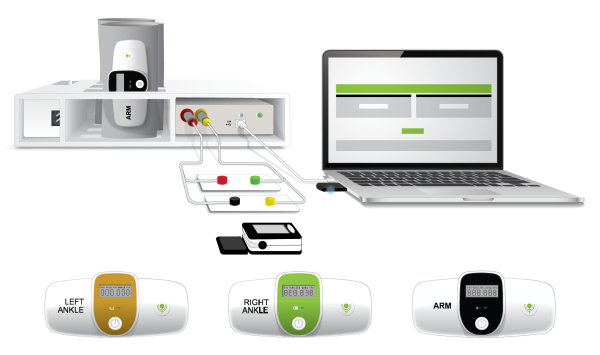
TM-FLOW
Distributor Company: Med-Essence Kft.
Address: 1222 Budapest, Orkán u. 11.
Website: www.e-med.hu
Manufacturer Company: LD Technology
Address: 100 N. Biscayne blvd. #502
33132 Florida, Miami
US
Website: http://www.ldteck.com/tm-flow
TM-Flow is a Medical Device Data System with the intended use of TBL-ABI Model C001, SweatC Model A001, and LD-Oxy Model D001.
· Measurement of Ankle Brachial Indices for the screening of Peripheral Artery Disease.
· Measurement of the Galvanic Skin response related to the sudomotor function.
· Mathematical Analysis of the Photoplethysmography for assessing:
. The Autonomic nervous system via Heart Rate Variability Analysis at rest and during the Ewing Tests.
. The Endothelial function via the Photoplethysmography (PTG) Analysis.
Clinical studies compared the accuracy of the TM-Flow:
· PTG Spectral Analysis Markers to the:
- The gold standard of Insulin resistance assessment
- Hyperinsulinemic Euglycemic Clamp.
- Coronary angiography.
- Vascular blood tests ( CRP and fibrinogen)
· Cardiometabolic Score to the Oral Glucose Tolerance Test (OGTT).
· Sudomotor markers to the:
- Small fiber Neuropathy Symptoms.
- Microvascular disease (retinopathy).
TM FLOW MAIN MARKERS
Vascular Function Assessment
Autonomic Nervous System Assessment